
Thoracic Umbrella Radiotherapy Study in stage IV NSCLC – TOURIST
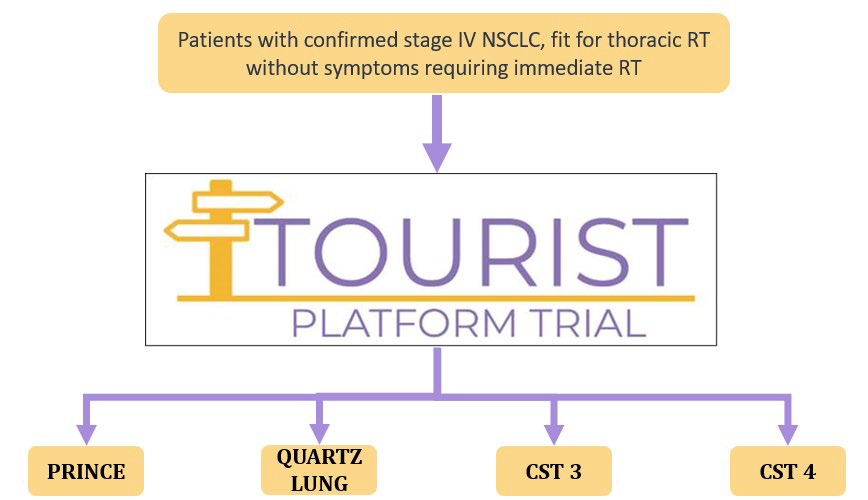

Prospective, randomised, multicentre trial of first line systemic treatment and radiotherapy in stage IV non-small cell lung cancer
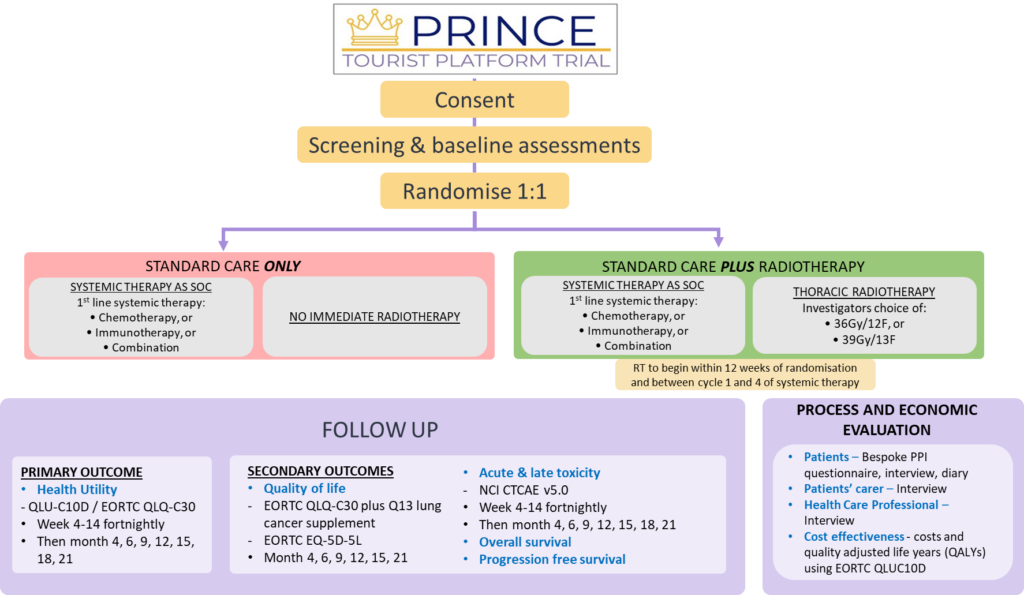
Trial Summary & Schema:
PRINCE is a multicentre, phase III, parallel group, randomised controlled trial with clinical and cost effectiveness, process evaluation and translational research. 472 participants, recruited over a 42-month period, will be randomised (1:1) to control or early high-dose palliative radiotherapy; all trial patients will receive first-line systemic therapy to determine the impact of the addition of early high-dose palliative thoracic radiotherapy on quality of life (QoL) in patients with stage IV NSCLC receiving first-line standard of care systemic therapy.
PRINCE RT QA Summary:
All QA activity will be streamlined with previous trial QA, where applicable. Please contact the RTTQA Group directly using the contact details below to discuss.
| QA Process | QA Activity | Required for Trial | Additional Details |
|---|---|---|---|
| Pre-Accrual | Facility Questionnaire | 1 Document filled in for both PRINCE and QUARTZ LUNG | |
| Outlining Benchmark Case | 1 Case | ||
| Planning Benchmark Case | 1 Case | ||
| Dummy Run | |||
| During Accrual | Individual Case Review | Retrospective: At least the 1st case submitted at each centre and 10% of total cases *Further prospective and/or timely retrospective reviews may be deemed necessary at the discretion of the RTTQA group and the trial CI. |
|
| Data collection | |||
| Dosimetry | Pre-accrual Lung or Spine SABR audits | All patients | |
| QA Streamlining | All activities | ADSCAN (+ other lung trials) |
RTTQA Contact: enh-tr.touristqa@nhs.net
Chief Investigator: Dr. David Woolf
PRINCE Candidate Chief Investigator: Professor Matthew Hatton
Trial Coordination Centre: Southampton Clinical Trials Unit (SCTU) tourist@soton.ac.uk
Sponsor: The Christie NHS Foundation Trust (the-christie.sponsoredresearch@nhs.net), PRINCE (tourist-prince@soton.ac.uk), QUARTZ LUNG (tourist-quartz@soton.ac.uk)
Funder: NIHR

Quality of life After Radiotherapy Treatment for patients with stage IV non-small cell Lung cancer
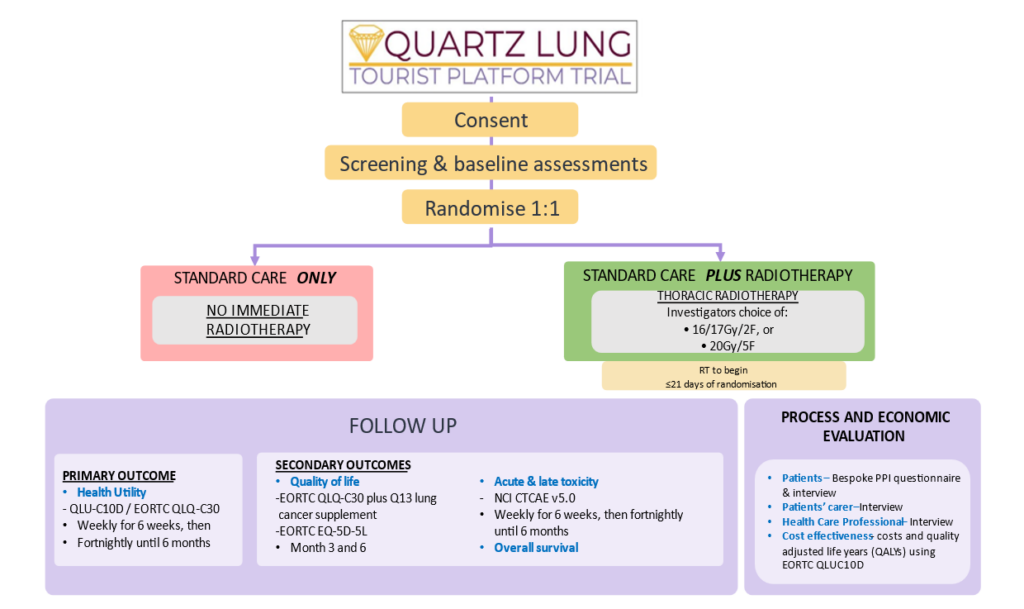
Trial summary and schema:
QUARTZ LUNG is a multi-centre phase III randomised control trial (RCT) with two treatment arms (1:1 randomisation). This trial is a CST within the TOURIST platform trial. 448 participants, recruited over a 42-month period, will be randomised (1:1) to the addition of thoracic radiotherapy to symptomatic palliative care to determine the clinical effectiveness of early low-dose palliative thoracic radiotherapy on overall survival, quality of life, lung cancer symptoms, acute and late toxicity. Follow up by patient-selected mode will be used for the PROMs data collection and to measure health utility with primary and secondary outcomes as listed in the main protocol document.
QUARTZ RT QA Summary:
All QA activity will be streamlined with previous trial QA, where applicable. Please contact the RTTQA Group directly using the contact details below to discuss.
| QA Process | QA Activity | Required for Trial | Additional Details |
|---|---|---|---|
| Pre-Accrual | Facility Questionnaire | 1 Document filled in for both PRINCE and QUARTZ LUNG | |
| Outlining Benchmark Case | 1 Case (*in cases where 2D applied field technique is not used for treatment) | ||
| Planning Benchmark Case | 1 Case (*in cases where 2D applied field technique is not used for treatment) | ||
| Dummy Run | *if a centre is using a 2D applied field treatment planning technique | ||
| During Accrual | Individual Case Review | Retrospective: At least the 1st case submitted at each centre and 10% of total cases *Further prospective and/or timely retrospective reviews may be deemed necessary at the discretion of the RTTQA group and the trial CI. |
|
| Data collection | |||
| Dosimetry | Pre-accrual Lung or Spine SABR audits | All patients | |
| QA Streamlining | All activities | ADSCAN (+ other lung trials) |
RTTQA Contact: enh-tr.touristqa@nhs.net
Chief Investigator: Dr. David Woolf
Trial Coordination Centre: Southampton Clinical Trials Unit (SCTU) tourist@soton.ac.uk
Sponsor: The Christie NHS Foundation Trust (the-christie.sponsoredresearch@nhs.net), QUARTZ LUNG (tourist-quartz@soton.ac.uk)
Funder: NIHR
